Details of the Drug
General Information of Drug (ID: DMJ4AWC)
| Drug Name |
Cyclothiazide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anhydron; Aquirel; Ciclotiazida; Ciclotiazide; Cyclothiazidum; Doburil; Fluidil; Renazide; Valmiran; Ciclotiazide [DCIT]; C 9847; MDi 193; Anhydron (TN); Ciclotiazida [INN-Spanish]; Cyclothiazidum [INN-Latin]; Lilly 35,483; Cyclothiazide [USAN:INN:BAN]; Cyclothiazide (JAN/USAN/INN); 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-chloro-3-(5-norbornen-2-yl)-, 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-chloro-3-(5-norbornen-2-yl)-,1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3-bicyclo(2.2.1)hept-5-en-2-yl-6-chloro-3,4-dihydro-, 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3-bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-3,4-dihydro-, 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-3-(5-norbornen-2-yl)-, 1,1-dioxide; 3-(bicyclo[2.2.1]hept-5-en-2-yl)-6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 3-Bicyclo(2.2.1)hept-5-en-2-yl-6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 3-Bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3,4-dihydro-3-(2-norbornen-5-yl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3,4-dihydro-3-(2-norbornen-5-yl)-2H-1,2-4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3,4-dihydro-3-(2-norbornen-5-yl)-7-sulfamoyl-1,2,4-benzothiadiazine 1,1-dioxide; 6-Chloro-3,4-dihydro-3-(5-norbornen-2-yl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3,4-dihydro-3-(norbornen-2-yl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3-(2-norbornen-5-yl)-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide; 6-chloro-3,4-dihydro-3-(norbornen-2-yl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
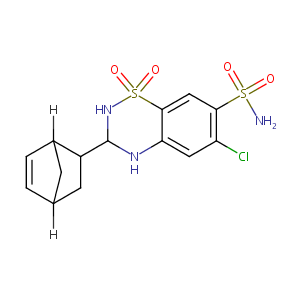 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 389.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Congestive heart failure | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
